
Medication Errors & Patient Safety – Part I
Why Medication Errors Matter More Than We Think
Medication errors are a significant public health concern, often occurring during various stages of care, whether preventive, diagnostic, therapeutic, or rehabilitative. These errors not only affect patient outcomes but also highlight critical gaps in the safe use of medicinal products. There is a growing need to strengthen risk mitigation strategies and enhance prevention efforts through existing regulatory mechanisms. Beyond the clinical impact, medication-related harm also places a substantial financial burden on healthcare systems globally, with associated costs estimated at around US $42 billion each year. Medication errors are unintended mistakes that can happen at any stage ofthe medication process, whether it’s during prescribing, storing, dispensing, preparation, or administration. When such errors occur repeatedly, follow a recognizable pattern, or lead to serious patient harm, it becomes critical to investigate the root causes and contributing factors. Understanding the clinical impact of these incidents, along with identifying practical solutions and preventive strategies, is key to ensuring they do notrecur.
Stages of Medication Use Process
- Storage
- Prescribing Stage
- Transcribing Stage
- Preparation Stage
- Dispensing Stage
- Administation Stage
- Monitoring Stage
What is a Medication Error?
A medication error is an unintended failure in the drug treatment process that leads to, or has the potential to lead to, harm to the patient.
Adverse Event:
GVP Annex I (Rev 3) defines an adverse event as any untoward medical occurrence in a patient or clinical trial subject administered a medicinal product and which does not necessarily have a causal relationship with this treatment. An adverse event can therefore be any unfavorable and unintended sign (including an abnormal laboratory finding, for example), symptom, or disease temporally associated with the use of a medicinal product, whether or not considered related to the medicinal product. Medication related adverse events should be distinguished from other adverse events (e.g. fall, surgery on wrong body site etc.).
Adverse Reaction:
An adverse reaction (ADR) is a response to a medicinal product which is noxious and unintended (Directive 2001/83/EC, Article 1 (11)). This includes adverse reactions which arise from:
- the use of a medicinal product within the terms of the marketing authorization.
- the use outside the terms of the marketing authorization, including overdose, off label use, misuse, abuse and medication errors;
- occupational exposure.
Patient Safety Incident
WHO’s Conceptual Framework for International Classification for Patient Safety (WHO ICPS) defines a patient safety incident as an event or circumstance that could have resulted, or did result, in unnecessary harm to a patient. The scope of patient safety incidents covers the entire health care process whereas the scope of (suspected) adverse reactions in pharmacovigilance is limited to the use of medicines by a consumer or healthcare professional. Patient safety incidents may occur in hospitals or other health care communities and may or may not involve a medicinal product.
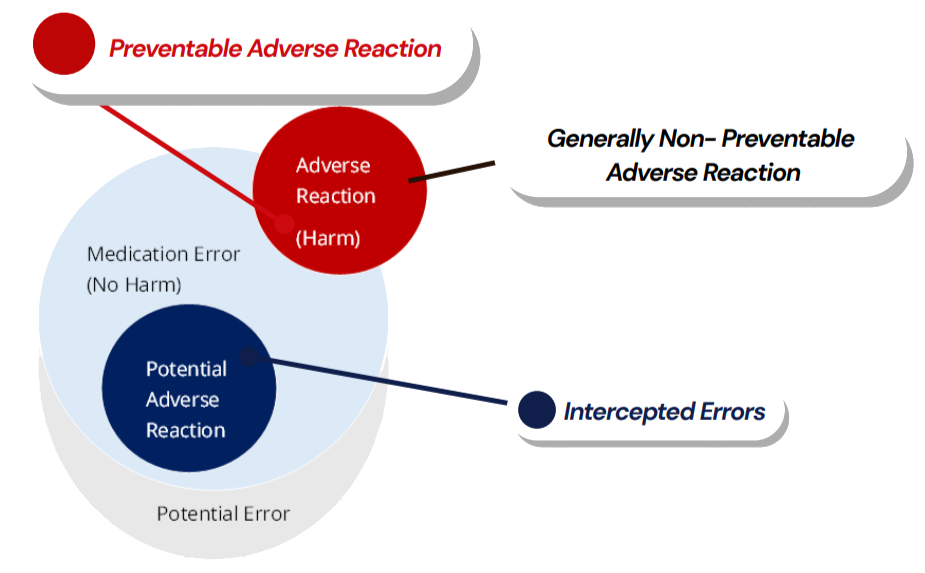
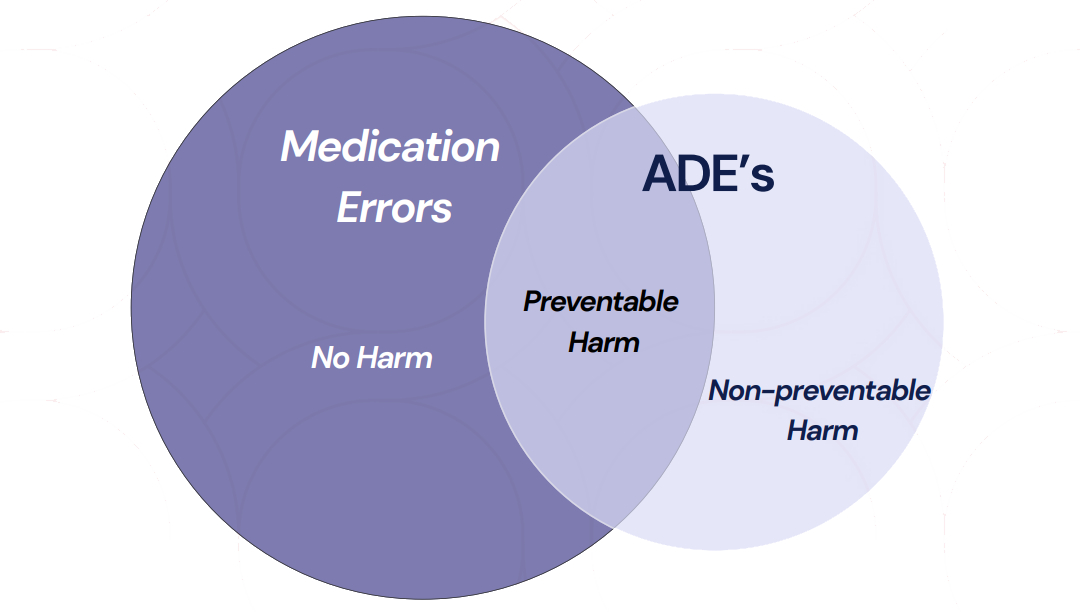
Correlation Among Medication Errors, Preventable and Non-Preventable Adverse Reactions, and Intercepted Errors
The diagram is provided for illustrative purposes only to support understanding of medication errors in the context of patient safety, and is not intended to inform or replace pharmacovigilance reporting obligations.
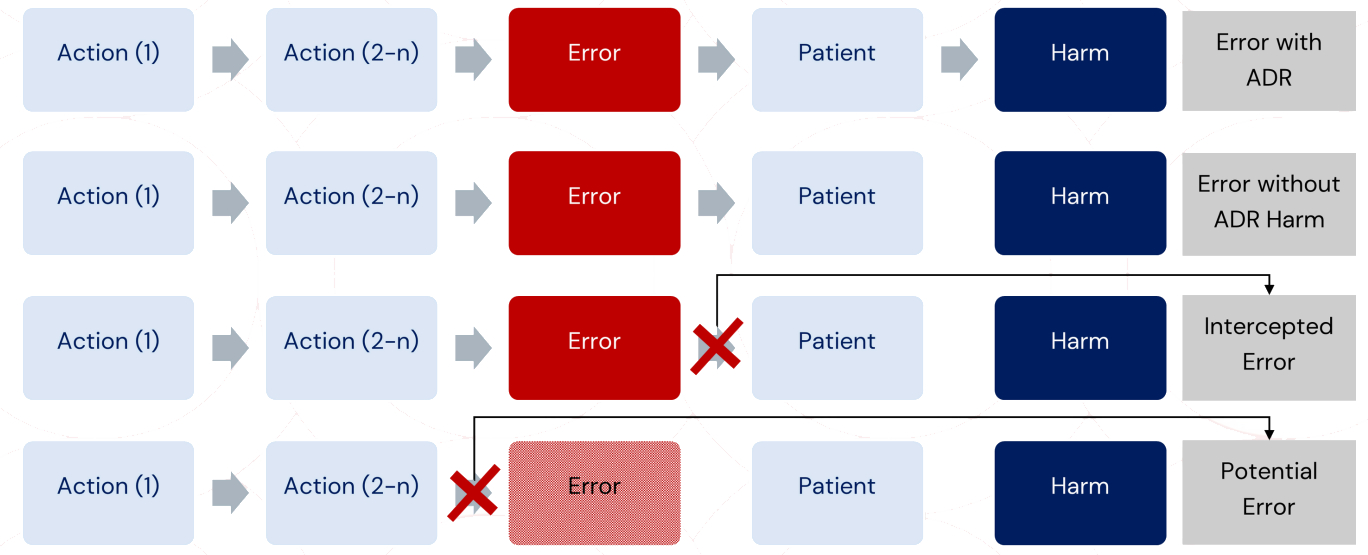
Classification of Medication Errors Reports
To support effective recording, coding, reporting, and assessment, medication errors should be classified based on factual information specific to each case.
Itis important to clearly distinguish between:
- Medication errors associated with adverse reaction(s)
- Medication errors without harm
- Intercepted medication errors
- Potential medication errors
The classification depends on where the break occurs in the sequence of events leading to the error and the resulting consequences forthe patient, as illustrated below:
 Ref: European Medicines Agency Good practice guide on recording, coding, reporting and assessment of medication errors. EMA/762563/2014
Ref: European Medicines Agency Good practice guide on recording, coding, reporting and assessment of medication errors. EMA/762563/2014
Intercepted Medication errors (Near Miss)
An intercepted error indicates that an intervention caused a break in the chain of events in the treatment process before reaching the patient which would have resulted in a ‘potential’ ADR. The intervention has prevented actual harm being caused to the patient. A near miss from a patient safety perspective is a random break in the chain of events leading up to a potential adverse event which has prevented injury, damage, illness or harm, but the potential for harm was nonetheless very near.
Example: A wrongly prepared medicine is intercepted by a nurse before being administered.
Potential medication errors
The recognition of circumstances that could lead to a medication error, and may or may not involve a patient. Refers to all possible mistakes in the prescribing, storing, dispensing, preparation for administration or administration of a medicinal product by all persons who are involved in the medication process and may lead to: a) medication error with harm, but without knowing the actual cause, b) medication error without harm and without knowing the actual cause, or c) medication error without harm, but with the awareness ofthe actual cause.
Example: Pharmacist noticed that the names of two medicines are similar and could clearly lead to product name confusion in practice, but no patient was actually involved or has taken the medicine.(Scenario c)
Medication Errors with Harm (Adverse Reactions)Medication errors that result in harm to the patient, specifically those associated with one or more adverse reactions. These cases also involve preventability.
Example: A patient receives an incorrect dose leading to hypotension and hospitalization.
Medication Errors without harm
NCC MERP Index for Categorizing Medication Errors
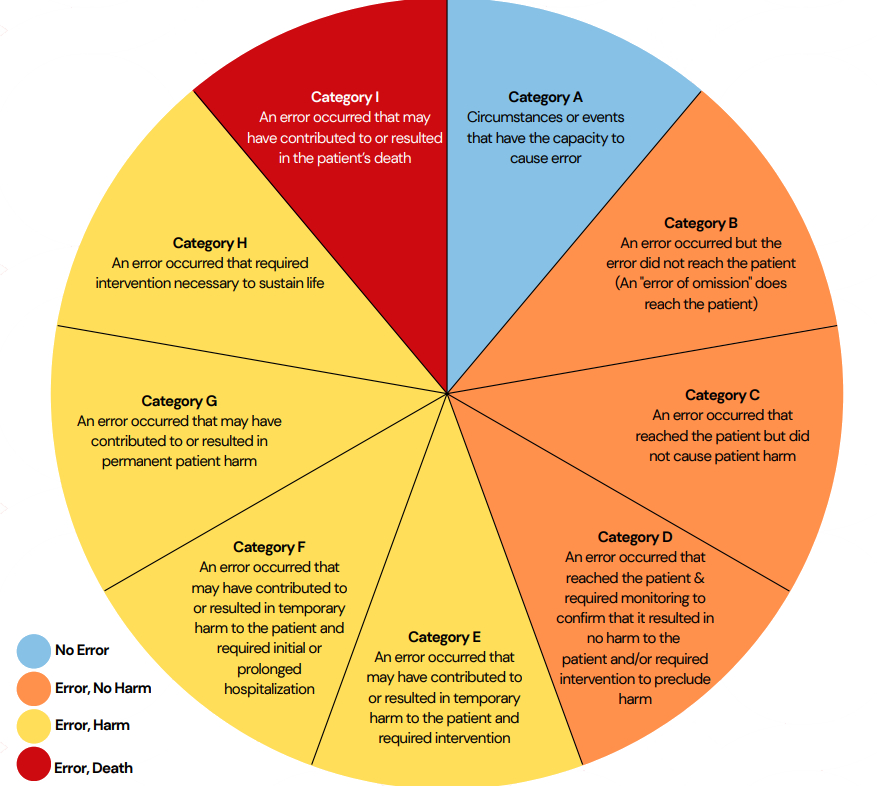
Ref:©2025 National Coordinating Council for Medication Error Reporting and Prevention. All Rights Reserved. *Permission is here by granted to reproduce information contained here in provided that such reproduction shall not modify the text and shall include the copyright notice appearing on the pages from which it was copied.
This copyright statement will change to the new year after the 1st of every year
References
1.Good practice guide on recording, coding, reporting and assessment of medication errors: 23 ctober 2015, EMA/762563/2014, Pharmacovigilance Risk Assessment Committee (PRAC).
2.Good practice guide on risk minimization and prevention of medication errors: 18 November 2015, EMA/606103/2014, Pharmacovigilance Risk Assessment Committee (PRAC)
3.Global burden of preventable medication-related harm in health care – A systematic review: WHO (World Health Organization)
4.MedDRA Coding of Medication Errors – General Principles
5.Product images taken from -Medication Errors: A CDER Perspective- Yelena Maslov, Pharm.D. (Team Leader), Division of Medication Error Prevention and Analysis Office of Medication Error Prevention and Risk Management Office of Surveillance and Epidemiology, June 25, 2015
6.National Coordinating Council for Medication Error Reporting and Prevention. Available at: www.nccmerp.org.
7.www.fda.com and www.ema.europa.eu
About Soterius
Soterius is a strong team of pharma professionals who design customized, innovative, and cost-efficient processes for clinical safety, pharmacovigilance, and medical affairs. Our deep industry knowledge and up to date insights let us combine agile, people powered intelligence in pioneering customer centric solutions. Our innovative technology solutions include engagement tools and communications platforms to create a unified and compliant medical access facility. With a strong global presence, we provide comprehensive clinical and post marketed safety services, that include aggregate report writing, signal detection and management, global literature surveillance, risk management, case processing and regulatory reporting.
We use state-of-the-art technologies to solve complex safety operations problems, be it case processing, intake, site reporting for clinical trials, or literature search and management. We have one of the most accurate solutions for case intake and case processing using AI.
We support companies from the initial development stage of a drug/vaccine to the approval and ultimate marketing of the therapy, supporting ongoing operations and regulatory commitments globally
Disclaimer
Copyright 2025 by Soterius, Inc. All rights reserved. Soterius logo are trademarks or registered trademarks of Soterius in all jurisdictions. Other marks may be trademarks or registered trademarks of their respective owners. The information you see, hear or read on the pages within this presentation, as well as the presentation’s form and substance, are subject to copyright protection. In no event, may you use, distribute, copy, reproduce, modify, distort, or transmit the information or any of its elements, such as text, images or concepts, without the prior written permission of Soterius. No license or right pertaining to any of these trademarks shall be granted without the written permission of Soterius (and any of its global offices and/or affiliates). Soterius reserves the right to legally enforce any infringement of its intellectual property, copyright and trademark rights.
Any content presented herewith should only be considered for general informational purposes and should not be considered as specific to the requirements of any particular organisation or for any specific purpose. Soterius, including its authors, presenters, and any affiliated individuals, does not make any representations or warranties about the completeness, reliability, appropriateness, relevance, or accuracy of the content presented here.
Please consult your physician/Health Care Provider for any matters related to health. No one should act on this information without specific professional advise.
Discover more
 abinash kumar •
10 Min Read
abinash kumar •
10 Min Read
Human Oversight in AI-Enabled Pharmacovigilance: What ‘HITL’ Actually Has to Mean
1. Why Human Oversight Is a Control, Not a Reassurance As artificial intelligence becomes embedded in pharmacovigilance workflows, the presence of a human reviewer is often assumed to be a…
 admin •
10 Min Read
admin •
10 Min Read
Deploying an Inspection Ready AI System in PV
Artificial intelligence in pharmacovigilance should not be assessed by how advanced the technology is. It should be assessed by what happens when the technology is wrong. Executive Summary Artificial intelligence…
 admin •
10 Min Read
admin •
10 Min Read
Is it Drug Induced Liver Injury (DILI), or something else?
Ever faced a hepatic signal in a trial that looked like classic drug-induced liver injury (DILI), but turned out to be something entirely different? Here’s a hypothetical scenario that might…
