
Navigating the Unique Challenges of AI and Automation in Pharmacovigilance: The Vital Role of Computer System Validation (CSV)
Artificial Intelligence, Machine Learning, Automation, Cost Reduction: all the buzzwords in pharmacovigilance! Everyone seems to be implementing Automation and AI in pharmacovigilance to reduce manual work and reduce costs of safety monitoring. However, compared to all other fields where Automation and AI is being implemented, pharma as an industry is facing a unique set of challenges in implementing these systems.
Regulators such the USFDA and EMA require that computerized systems should be fit for intended use and meet current regulatory requirements. ‘Fit for intended use’ is a broad term that encompasses detailed testing, documentation, qualification and validation activities to demonstrate that the ‘use’ and ‘fitness for such use’ of a system is demonstrated effectively and is available for review during audits and inspections.
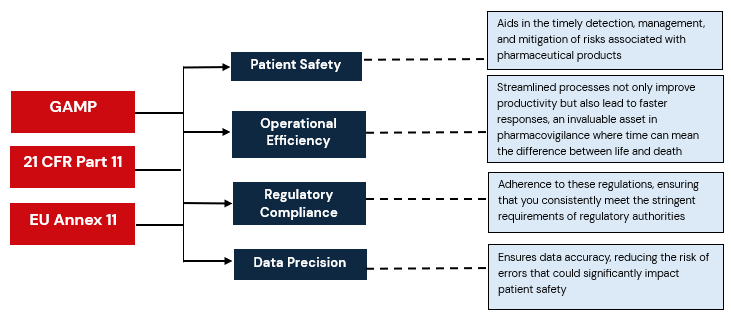
The method for achieving this is Computer System Validation, which is rooted in the principles of Good Automated Manufacturing Practice (GAMP), Title 21 CFR Part 11, and EU Annex 11. In the pharma space, the requirements become all the more critical, since it is no longer just about compliance; it’s about safeguarding lives.
The Indispensable role of CSV in Pharmacovigilance
| End Point | Regulation | Requirement | How CSV Helps to Achieve the Requirement |
| Patient Safety | EU Annex 11 | Patient Safety | Aids in the timely detection, management, and mitigation of risks associated with pharmaceutical products |
| Data Precision | GAMP | Precise Reporting | Ensures data accuracy, reducing the risk of errors that could significantly impact patient safety. |
| Regulatory Compliance | Title 21 CFR Part 11, EU Annex 11 | Robust compliance standards for
electronic records and signatures in FDA-regulated industries, guidelines For computerized systems in the European Union |
Adherence to these regulations, ensuring that you consistently meet the stringent requirements of regulatory authorities |
| Operational Efficiency | GAMP, Title 21 CFR, and EU Annex 11 | Importance of efficient systems | Streamlined processes not only improve productivity but also lead to faster responses, an invaluable asset in pharmacovigilance where time can mean the difference between life and death |
In the next few parts of this series, I will be discussing some approaches that we have followed in implementing CSV procedures for software systems developed for the pharmacovigilance space, what we have learned in the process, how CSV can be applied by harmonizing international regulatory requirements and how it can
be applied to the agile development. Stay tuned for more updates and reach out to me if you would like to see any other topics covered as part of the series.
About Soterius
Soterius is a strong team of pharma professionals who design customized, innovative, and cost-efficient processes for clinical safety, pharmacovigilance, and medical affairs. Our deep industry knowledge and up to date insights let us combine agile, people powered intelligence in pioneering customer centric solutions. Our innovative technology solutions include engagement tools and communications platforms to create a unified and compliant medical access facility. With a strong global presence, we provide comprehensive clinical and post marketed safety services, that include aggregate report writing, signal detection and management, global literature surveillance, risk management, case processing and regulatory reporting. We use state-of-the-art technologies to solve complex safety operations problems, be it case processing, intake, site reporting for clinical trials, or literature search and management. We have one of the most accurate solutions for case intake and case processing using AI.
We support companies from the initial development stage of a drug/vaccine to the approval and ultimate marketing of the therapy, supporting ongoing operations and regulatory commitments globally.
Disclaimer
Copyright 2025 by Soterius, Inc. All rights reserved. Soterius logo are trademarks or registered trademarks of Soterius in all jurisdictions. Other marks may be trademarks or registered trademarks of their respective owners. The information you see, hear or read on the pages within this presentation, as well as the presentation’s form and substance, are subject to copyright protection. In no event, may you use, distribute, copy, reproduce, modify, distort, or transmit the information or any of its elements, such as text, images or concepts, without the prior written permission of Soterius. No license or right pertaining to any of these trademarks shall be granted without the written permission of Soterius (and any of its global offices and/or affiliates). Soterius reserves the right to legally enforce any infringement of its intellectual property, copyright and trademark rights. Any content presented herewith should only be considered for general informational purposes and should not be considered as specific to the requirements of any particular organisation or for any specific purpose. Soterius does not make any representations or warranties about the completeness, reliability, appropriateness, relevance, or accuracy of the content presented here.
Confidential Information
Copyright@2025
Soterius, Inc.
Discover more
 abinash kumar •
10 Min Read
abinash kumar •
10 Min Read
Human Oversight in AI-Enabled Pharmacovigilance: What ‘HITL’ Actually Has to Mean
1. Why Human Oversight Is a Control, Not a Reassurance As artificial intelligence becomes embedded in pharmacovigilance workflows, the presence of a human reviewer is often assumed to be a…
 admin •
10 Min Read
admin •
10 Min Read
Deploying an Inspection Ready AI System in PV
Artificial intelligence in pharmacovigilance should not be assessed by how advanced the technology is. It should be assessed by what happens when the technology is wrong. Executive Summary Artificial intelligence…
 admin •
10 Min Read
admin •
10 Min Read
Is it Drug Induced Liver Injury (DILI), or something else?
Ever faced a hepatic signal in a trial that looked like classic drug-induced liver injury (DILI), but turned out to be something entirely different? Here’s a hypothetical scenario that might…
