
Causality Assessment in Pharmacovigilance
The causality assessment of adverse events, to determine the relationship or connection between the drug and adverse events, is an essential and complex approach in pharmacovigilance. The recognition of a potential safety issue for a drug requires adverse drug reactions to be readily differentiated from adverse events.
An adverse drug reaction is distinguished from an adverse event by the fact that in an adverse drug reaction, a causal relationship is suspected between a drug and an adverse event. Hence, all cases assessed by either the reporting healthcare professional or the sponsor as having a reasonable suspected causal relationship to the drug qualify as adverse drug reactions.
For the purposes of regulatory reporting, if an adverse event is reported spontaneously, even if the relationship is unknown, it meets the criteria of an adverse drug reaction. Hence, all spontaneous reports reported by healthcare professionals or consumers are considered suspected adverse drug reactions since they denote the suspicion of the primary sources, unless the reporters specifically mentions that that a causal relationship can be excluded, or they consider the events to be unrelated.
Need for Causality Assessment
A fundamental issue in pharmacovigilance is that many cases concern suspected adverse drug reactions. In real-life situations, a very limited number of adverse reactions qualify as ‘certain’ or ‘unlikely’; most are usually in between, i.e., either ‘possible’ or ‘probable’. To address this issue, many methods have been developed to harmonize causality assessment. However, causality assessment has become a common routine activity in pharmacovigilance.
The advantages of causality assessment include the following:
- Provides uniformity and reduce disagreement between reviewers
- Provides likelihood of relationship
- Mark individual cases
- Improves case evaluation and benefit-risk assessment
Methods of Causality Assessment
There are numerous methods published for causality assessment of adverse events. These fall into the following 3 broad categories: Expert judgement/Global introspection, Algorithms and Probabilistic methods (Bayesian approaches):
| Categories of Causality Assessment Methods | |||
| Expert Judgement / Global Introspection | Algorithms | Probabilistic Methods | |
| Examples | WHO UMC causality assessment | Naranjo Scale | Bayesian Approaches |
| Feature | Individual assessments are performed based on clinical experience and previous knowledge using no standardized tool to arrive at causality conclusion. | Sets of specific questions with associated scores for calculating the likelihood of a causal relationship. | Specific findings in a case are used to transform the prior estimate of probability into a posterior estimate of probability of drug causation. The prior probability is determined from epidemiological information, and the posterior probability combines this background information with the individual case evidence to deduce the estimate of causation. |
The 2 commonly accepted and used methods for causality assessment across the globe are the following:
WHO UMC causality assessment
A method developed by World Health Organization (WHO) and Upsala Monitoring centre (UMC) at Sweden as a practical instrument for the assessment of causal relationship. This is a combined assessment considering the clinical-pharmacological aspects of the case and the quality of documentation of the observation.
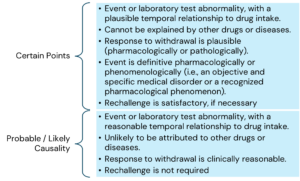
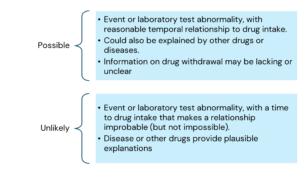
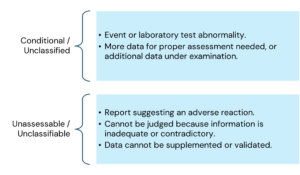
The main criteria for causality assessment in this method includes temporal relationships between the drug and the adverse event; absence of other confounding factors (e.g., drugs, underlying disease etc.); response to drug withdrawal (DE challenge); and response to drug re-administration (rechallenge).
The various causality categories include the following:
Here is the information arranged by topic with the points listed underneath:
Naranjo causality assessment (Naranjo Scale)
The Naranjo algorithm (Adverse Drug Reaction (ADR) Probability Scale) was developed by Naranjo and coworkers in 1991 to determine the possibility of whether an ADR is due to the drug rather than due to other contributory factors. The probability is assigned using a simple questionnaire to assign scores. There are 10 questions in the questionnaire scale that are answered as either “Yes”, “No”, or “Do not know”. Different point values (-1, 0, +1 or +2) are assigned to each answer.
The total scores in the actual ADR Probability Scale range from -4 to +13; the reaction is considered definite when the score is 9 or higher, probable between 5 to 8, possible between 1 to 4, and doubtful if 0 or less.
Conclusion
Causality assessment to assess the relationship or connection between the drug and adverse events is a key component for benefit-risk assessment and identification / assessment of safety signals. Despite various methods developed and standardized, no specific method is accepted universally, although the expert judgement/global introspective method is most commonly used, as algorithm-based and probabilistic methods have been shown to be tough to reliably implement in real situations.
References
- ICH Topic E2A Clinical Safety Data Management: Definitions and Standards for Expedited Reporting: European Medicines Agency: June 1995
- The use of the WHO-UMC system for standardised case causality assessment; 06 April 2018. (who-umc-causalityassessment_new-logo.pdf)
- Management of Safety Information from Clinical Trials: Report of CIOMS Working Group VI: Geneva 2005
- Guideline on good pharmacovigilance practices (GVP) Module VI – Management and reporting of adverse reactions to medicinal products: European Medicines Agency: November 20172
- Agbabiaka TB, Savović J, Ernst E. Methods for causality assessment of adverse drug reactions: a systematic review. Drug Saf. 2008;31(1):21-37. doi: 10.2165/00002018-200831010-00003. PMID: 18095744.
- Renata Rezende de Menezes et al: Causality assessment of adverse drug reactions by applying a global introspection method
- in a high complexity hospital: Exploratory Research in Clinical and Social Pharmacy: Volume 3, September 2021, 100064
- Martin J. Doherty: Algorithms for assessing the probability of an Adverse Drug Reaction: Respiratory Medicine CME Volume 2, Issue 2, 2009, Pages 63-67
About Soterius
Soterius is a strong team of pharma professionals who design customized, innovative, and cost-efficient processes for clinical safety, pharmacovigilance, and medical affairs. Our deep industry knowledge and up to date insights let us combine agile, people powered intelligence in pioneering customer centric solutions. Our innovative technology solutions include engagement tools and communications platforms to create a unified and compliant medical access facility. With a strong global presence, we provide comprehensive clinical and post marketed safety services, that include aggregate report writing, signal detection and management, global literature surveillance, risk management, case processing and regulatory reporting. We use state-of-the-art technologies to solve complex safety operations problems, be it case processing, intake, site reporting for clinical trials, or literature search and management. We have one of the most accurate solutions for case intake and case processing using AI.
We support companies from the initial development stage of a drug/vaccine to the approval and ultimate marketing of the therapy, supporting ongoing operations and regulatory commitments globally.
