Drug Induced Liver Injury in Premarketing Clinical Trials
DILI (Drug Induced Liver Injury) is a rare but potentially fatal adverse drug reaction.
DILI is a very common cause of acute liver failure in North America and Europe, a key reason for the failure of a drug to obtain marketing authorization, and a common cause of post-marketing restrictions and product withdrawals.
The evaluation of DILI is critical because most drugs that cause severe DILI do so infrequently and usual drug development databases with up to a few thousand subjects exposed to a new drug will not reveal any cases.
Such databases, on the other hand, may show evidence or signals of a drug ‘ s potential for severe DILI if clinical and laboratory data are properly assessed for evidence of lesser injury that may not be severe but could predict the ability to cause more severe injuries.
For the past 50 years, DILI has been a very common cause of safety-related drug marketing withdrawals.
Here are some examples of such drug withdrawals:
- Tolcapone, troglitazone, trovafloxacin, bromfenac, nefazodone, lumiracoxib and sitaxentan.
- Drugs that were not approved in the United States because their hepatotoxicity was identified during European marketing – ibufenac, perhexiline, alpidem.
- Drugs that were not approved in the United States because premarketing data revealed the possibility of severe DILI: dilevalol, tasosartan, ximelagatran.
Mechanism and Types of DILI
Drugs can cause liver injuries through varied mechanisms. These injuries are similar to almost all known liver diseases, and there are no pathognomonic findings, even on liver biopsy, that confirm a diagnosis of DILI
DILI can look like almost any type of acute or chronic liver disease. Hence, when DILI is suspected, additional clinical and laboratory information should be obtained for differential diagnosis of the cause. The mechanisms and the risk factors for DILI are poorly understood in most cases.
Despite their rarity, both idiosyncratic and indirect DILI can lead to severe and sometimes fatal liver injury.
Most of the drugs withdrawn from the market for hepatotoxicity have caused death or transplantation at frequencies in the range of ≤1 per 10,000, so that a single case of such an event rarely would be found even if several thousand subjects were studied.
Severe DILI cases rarely have been seen in drug development programs of significantly hepatotoxic drugs.
DILI reactions are usually categorized as follows:
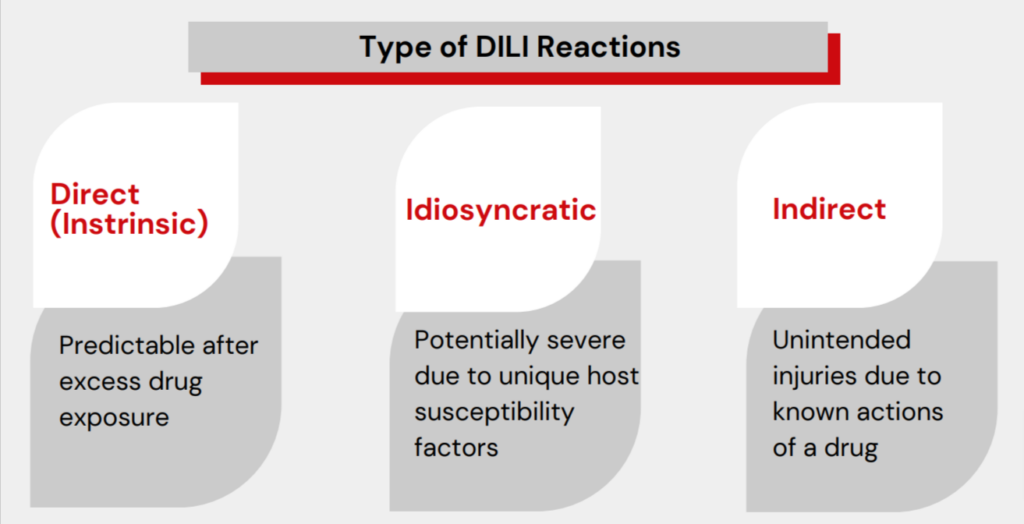
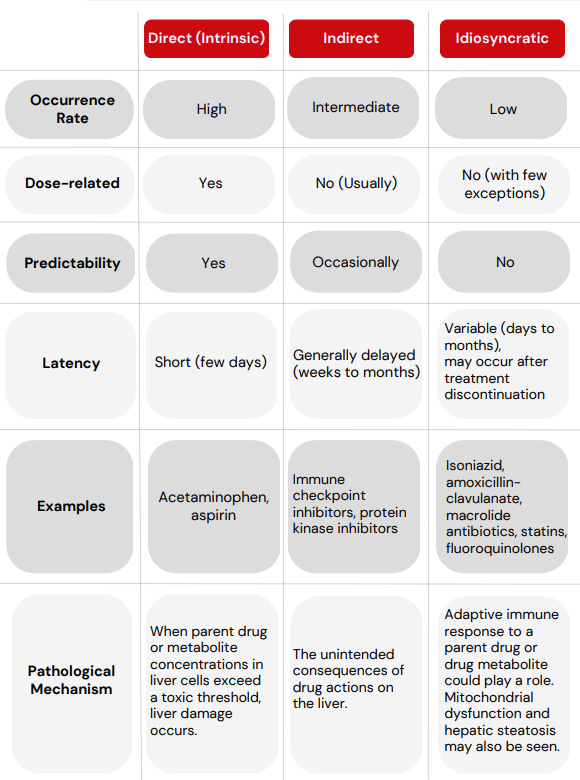
DILI Categories
Signals of DILI and Hy’s Law
Hy’s Law is essentially a translation of Zimmerman’s finding that hyperbilirubinemia
caused by pure hepatocellular injury is an ominous sign of a drug’s potential to
cause substantial liver injury.
In a summary, Hy‘s Law cases consist of the following three elements:
- The drug causes hepatocellular injury, generally shown by a higher incidence of 3-fold or greater elevations above the ULN of aminotransferases (ATs) ALT or AST than the (nonhepatotoxic) control drug or placebo.
- Among trial subjects showing such AT elevations, often with ATs much greater than 3xULN, one or more also show elevation of serum Total bilirubin (TBL) to >2xULN, without initial findings of cholestasis (elevated serum ALP).
- No other reason can be found to explain the combination of increased AT and TBL, such as viral hepatitis A, B, or C; preexisting or acute liver disease; or another drug capable of causing the observed injury.
According to Hy‘s law, there is a 10–50% risk that a patient will develop acute liver failure (ALF), which can lead to death or liver transplantation.
The presence of even one or two such cases in a clinical trial programme is significant because it indicates a higher risk for idiosyncratic ALF in a postmarket population treated with the same medication under equivalent conditions
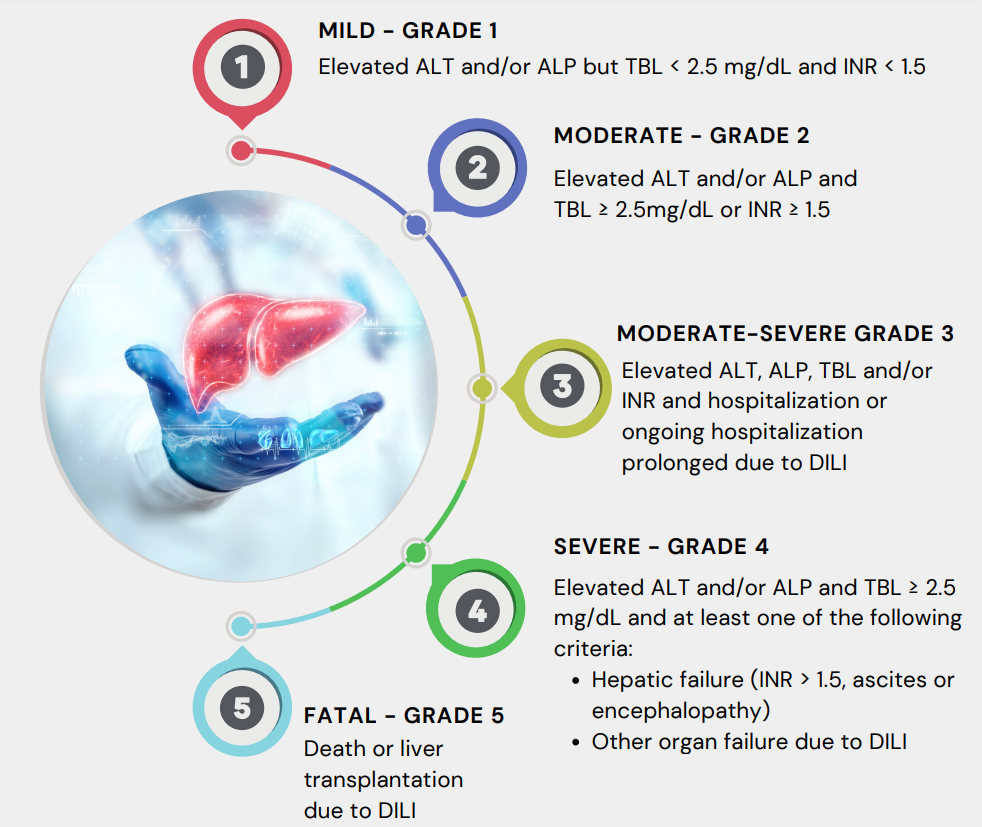
Severity of DILI
The National Cancer Institute’s grading system of Common Toxicity Criteria for Adverse Events (NCI-CTCAE) may be useful for signal detection and identification of changes in liver tests at the individual and aggregate levels in a clinical study. However, these criteria do not specifically correlate with hepatocellular function or clinical outcome. These criteria were not developed specifically for DILI, and their severity grading is not stratified by risk levels
For the assessment of post-marketing cases of suspected DILI, a five-level categorical scale with specified clinical and laboratory test results has been used by the NIH Drug Induced Liver Injury Network (DILIN) and the United States Food and Drug Administration (U.S. FDA). The same is mentioned below:
DILI is challenging to predict during the drug development process. The underlying mechanism of DILI are incompletely understood.
For intrinsic DILI, some of the potential risks can be flagged by preclinical models and in vitro test systems, but these are not very useful in assessing the risk of idiosyncratic DILI.
Considering severe DILI is generally rare, finding one case may require the treatment of thousands of people from diverse patient populations.
Due to the limited number of clinical trial subjects, monitoring the standard serum liver tests to detect milder liver injury can be considered the predominant approach to predict the risk of possible DILI in clinical trials.
Considering that there may be varied mechanisms of DILI and different clinicopathological phenotypes, a systematic collection of adequate diagnostic datasets along with a focused causality assessment performed by clinical professionals having expertise in this area is required for the evaluation of each potential case of DILI in clinical trials.
References
- Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov 2005;4:489-99.
- Kullak-Ublick GA,Andrade RJ, Merz M, End P, Benesic A, Gerbes AL, et al. Drug induced liver injury: recent advances in diagnosis and risk assessment. Gut 2017;66:1154-1164.
- Davern TJ, Chalasani N. Drug induced liver injury in clinical trials: as rare as hens’ teeth. Am J Gastroenterol. 2009;104:1159-61.
- Aithal GP, Watkins PB, Andrade RJ, et al. Case definition and phenotype standardization in drug induced liver injury. Clin Pharmacol Ther 2011;89:806-15.
- Watkins PB, Seligman PJ, Pears JS, Avigan MI, Senior JR. Using controlled clinical trials to learn more about acute drug induced liver injury. Hepatology 2008; 48(5):1680-1689.
- Drug induced liver injury (DILI): Current status and future directions for drug development and the post-market setting. A consensus by a CIOMS Working Group. Geneva, Switzerland: Council for International Organizations of Medical Sciences (CIOMS), 2020.
- Guidance for Industry Drug Induced Liver Injury: Premarketing Clinical Evaluation. U S. Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER). Center for Biologics Evaluation and Research (CBER). July 2009. Drug Safety.
About Soterius
Soterius is a strong team of pharma professionals who design customized, innovative, and cost-efficient processes for clinical safety, pharmacovigilance, and medical affairs. Our deep industry knowledge and up to date insights let us combine agile, people powered intelligence in pioneering customer centric solutions. Our innovative technology solutions include engagement tools and communications platforms to create a unified and compliant medical access facility. With a strong global presence, we provide comprehensive clinical and post marketed safety services, that include aggregate report writing, signal detection and management, global literature surveillance, risk management, case processing and regulatory reporting. We use state-of-the-art technologies to solve complex safety operations problems, be it case processing, intake, site reporting for clinical trials, or literature search and management. We have one of the most accurate solutions for case intake and case processing using AI.
We support companies from the initial development stage of a drug/vaccine to the approval and ultimate marketing of the therapy, supporting ongoing operations and regulatory commitments globally.

