
Postmarketing Adverse Drug Experience (PADE) Inspections – Part I
Legal Framework of PADE Inspections
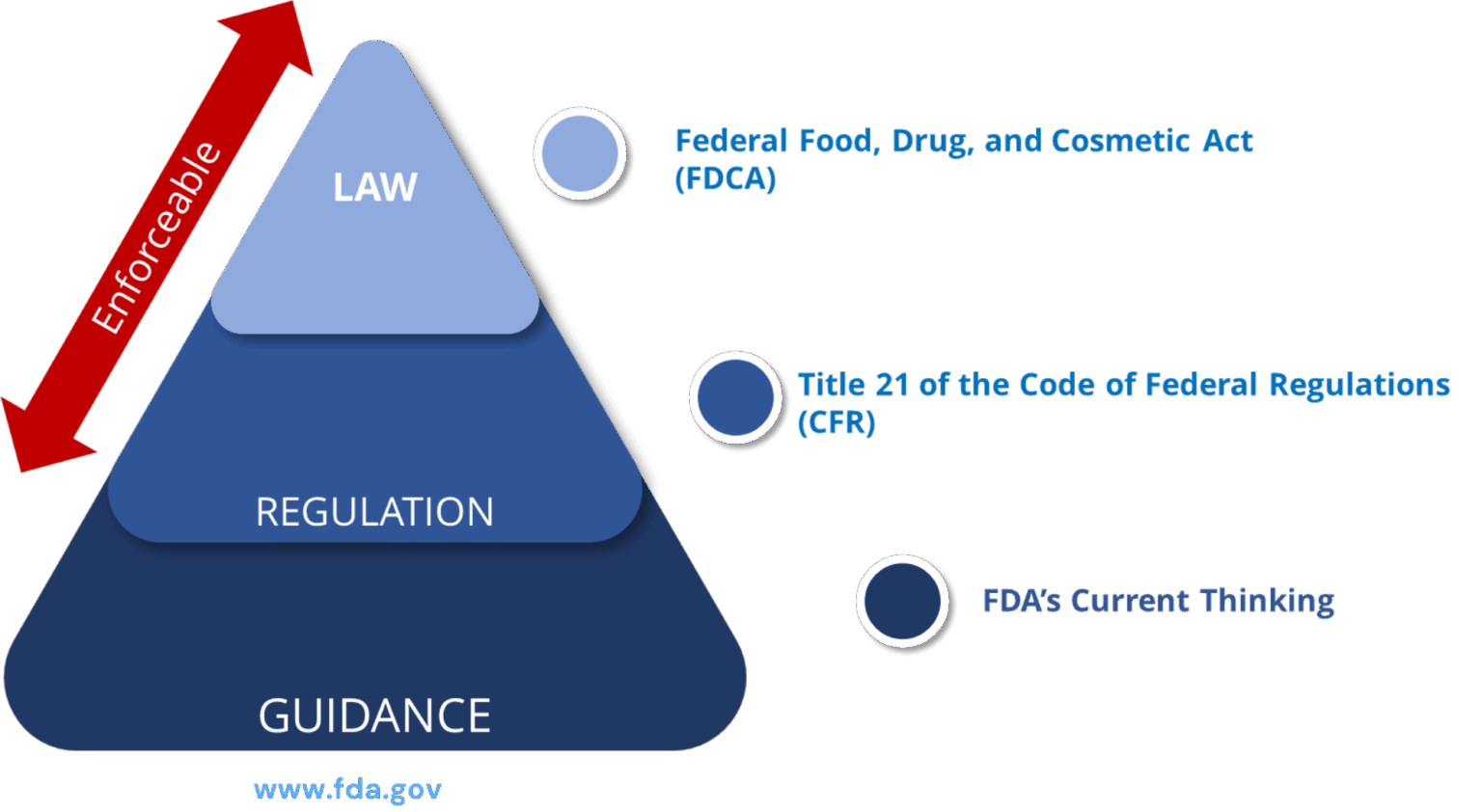
PADE Statutory Provisions / Regulations: Prescription Drug Products for Human Use
| S.no | FD&C Act, subchapter V, part A, section 505 (21 U.S.C. 355) | Comments |
| 1 | 21 CFR 310.305 | New Drugs: Records and reports concerning adverse drug experiences (ADEs) for marketed prescription drugs for human use without an approved new drug application |
| 2 | 21 CFR 314.80 | New drug applications: Post marketing reporting of ADEs |
| 3 | 21 CFR 314.81(b)(2) | New drug applications: Annual reports |
| 4 | 21 CFR 314.90 | New drug applications: Waivers |
| 5 | 21 CFR 314.98 | Abbreviated applications: Post marketing reports |
| 6 | 21 CFR 314.540 | Accelerated approval of new drugs for serious or life-threatening illnesses: Post marketing safety reporting |
| 7 | 21 CFR 314.630 | Approval of new drugs when human efficacy studies are not ethical or feasible: Post marketing safety reporting |
| 8 | 21 CFR part 4, subpart B | Post marketing safety reporting for combination products |
Approval vs. Marketing
Once a drug is approved, applicant holders MUST receive, evaluate, and report adverse drug experiences (ADEs) to FDA, even if the drug is not marketed.

PADE Inspection – Scope
- Written procedures
- Product list (approval date, status, etc.)
- Late or Missing Periodic Reports or Annual Reports
- Late, missing, incomplete, or inaccurate 15-day reports
- ADEs from all sources
- Root cause analyses and corrective actions for deviations
- Confirmations for electronic submissions
- Training Documents
- Safety Contracts, Agreements, and Business Partners
- Organization, roles, and responsibilities
- Waivers
Who can be inspected for PADE Compliance?
-
Application holders:
Applicants with approved drugs and therapeutic biologics (prescription and non-prescription)
- New Drug Application (NDA)
- Abbreviated New Drug Application (ANDA)
- Biologics License Application (BLA)
-
Non- Applicants:
Manufacturers, packers, distributors, retailers, and certain others named on product labels (responsibilities vary based on product type)
- Approved prescription and non- prescription drugs and therapeutic biologics (NDA, ANDA, BLA)
- Unapproved prescription drugs
- Unapproved non-prescription drugs
-
Third parties:
Contractors, vendors, and other third parties
- Pharmacovigilance activities conducted on behalf of application holders or non-applicants
Risk Based Selection for PADE Inspection
-
Inspection History:
-
Compliance and inspection history
-
-
-
- Never inspected for PADE compliance
- Inspection findings from other program areas
-
-
- Firm’s written responses to previous PADE inspections
-
Firm Information:
- Corporate changes
- Portfolio (type and number of products)
- Complaints
- Internal FDA information
- Information from other health authorities
-
Product Portfolio:
- New molecular entities
- High-risk
- Patient exposure
- Recalls Submissions to FDA
-
-
- Individual Case Safety Reports (ICSRs)
- Annual reports
- Periodic report
-
References
- https://www.fda.gov/
- Number of 483 issued from the System* Inspections ending between 10/1/2022 and 9/30/2023 https://www.fda.gov/inspections-compliance-enforcement-and- criminal-investigations/inspection-references/inspection-observations
- Post marketing Drug Safety Compliance: 2019 Inspection Findings April 29, 2020 (Live Webinar) Centre for Drug Evaluation and Research – Small Business and Industry Assistance, Centre for Drug Evaluation and Research, US Food and Drug Administration
- Postmarketing Drug Safety and Inspection Readiness June 19, 2018 Centre for Drug Evaluation and Research (CDER) Small Business and Industry Assistance (SBIA) Webinar
- Post marketing Drug Safety and Inspection Readiness June 19, 2018 Centre for Drug Evaluation and Research (CDER) Small Business and Industry Assistance (SBIA) Webinar
- CHAPTER 53 – Post marketing Surveillance and Epidemiology: Human Drug and Therapeutic Biological Products, fda.gov
About Soterius
Soterius is a strong team of pharma professionals who design customized, innovative, and cost-efficient processes for clinical safety, pharmacovigilance, and medical affairs. Our deep industry knowledge and up to date insights let us combine agile, people powered intelligence in pioneering customer centric solutions. Our innovative technology solutions include engagement tools and communications platforms to create a unified and compliant medical access facility. With a strong global presence, we provide comprehensive clinical and post marketed safety services, that include aggregate report writing, signal detection and management, global literature surveillance, risk management, case processing and regulatory reporting. We use state-of-the-art technologies to solve complex safety operations problems, be it case processing, intake, site reporting for clinical trials, or literature search and management. We have one of the most accurate solutions for case intake and case processing using AI.
We support companies from the initial development stage of a drug/vaccine to the approval and ultimate marketing of the therapy, supporting ongoing operations and regulatory commitments globally.
