
Hy’s law & Drug Induced Liver Injury – Part II
Drug-Induced Liver Injury Occurrence
- Most frequent cause of acute liver failure in North America and Europe.
- No definite causative agent has been attributed in several cases.
- Underlying mechanisms are still unclear and hence is difficult to predict during drug development.
- May mimic almost any known type of liver disease.
- Rare yet potentially life-threatening.
- Key reason for drugs to fail to achieve marketing authorization, frequent cause for post-authorization restrictions and product withdrawals.
- Monitoring of standard serum liver tests to detect milder liver injury is the main approach to anticipate a possible DILI risk in Clinical Trials.
- Evaluation of each potential DILI case in clinical trials requires a systematic collection of adequate diagnostic datasets and a rigorous assessment for causality, performed by clinical experts in this area.
- The evaluation of DILI is critical because most drugs that cause severe DILI do so infrequently and usual drug development databases with up to a few thousand subjects exposed to a new drug will not reveal any cases.
- Such databases, on the other hand, may show evidence or signals of a drug’s potential for severe DILI, if clinical and laboratory data are properly assessed for evidence of lesser injury, that may not be severe but could predict the ability to cause more severe injuries.
FDA’ s eDISH Program for Hepatotoxicity Assessment
Hy’s Law and eDISH Development: The Hy’s Law principle served as the foundation for the FDA’s creation of the ‘eDISH’ software program, designed to evaluate Drug-Induced Serious Hepatotoxicity.
Step-Based Approach:
- Data from case reports are examined for peak values of liver enzymes ALT and TBL over the observation period. These values are plotted on an x-y chart as logarithm10 multiples of elevations above the upper limits of the normal reference ranges (ULN).
- For an individual patient, time course of ALT, TBL, AST, and ALP are plotted together for visual comparison.
- A medical text narrative, written by a skilled physician, provides additional context about the patient’s condition. This narrative helps estimate the most likely cause of abnormal findings and assesses the probability of drug-induced hepatotoxicity.
Causality Assessment:
Requires considering of multiple potential factors.
Several possible causes are common and Insufficient to simply label cases as ‘confounded.’
Estimated likelihood is categorized as ‘probable’ if the likelihood is over 50% and higher than all other causes combined.
Sufficient information and thorough patient investigation are essential to rule out alternative causal factors.
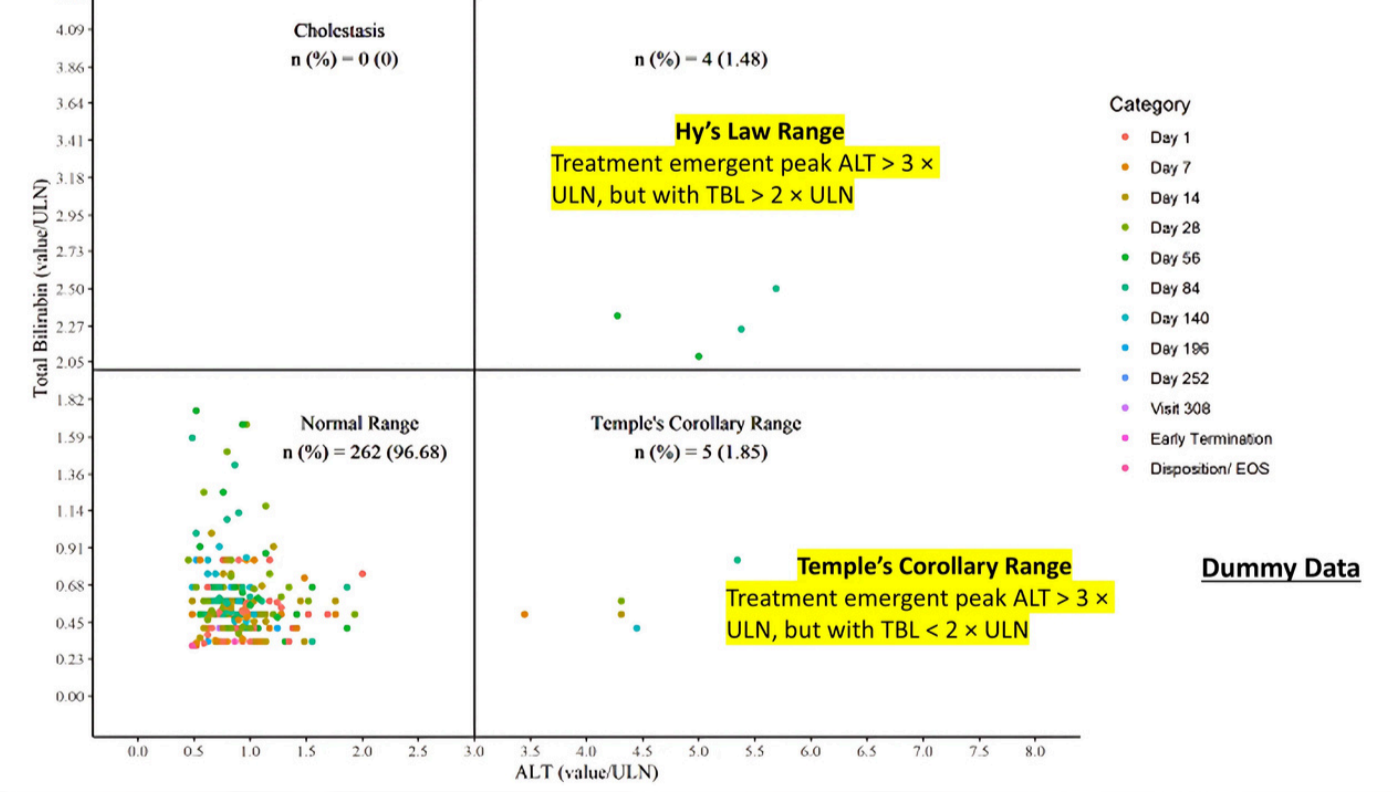
Note: The upper right quadrant doesn’t automatically define cases as ‘Hy’s Law’; It identifies patients as of unique importance. Further clinical information is essential for a comprehensive medical diagnosis aimed at identifying the most likely cause of the observed findings.
Approach to the diagnosis of DILI
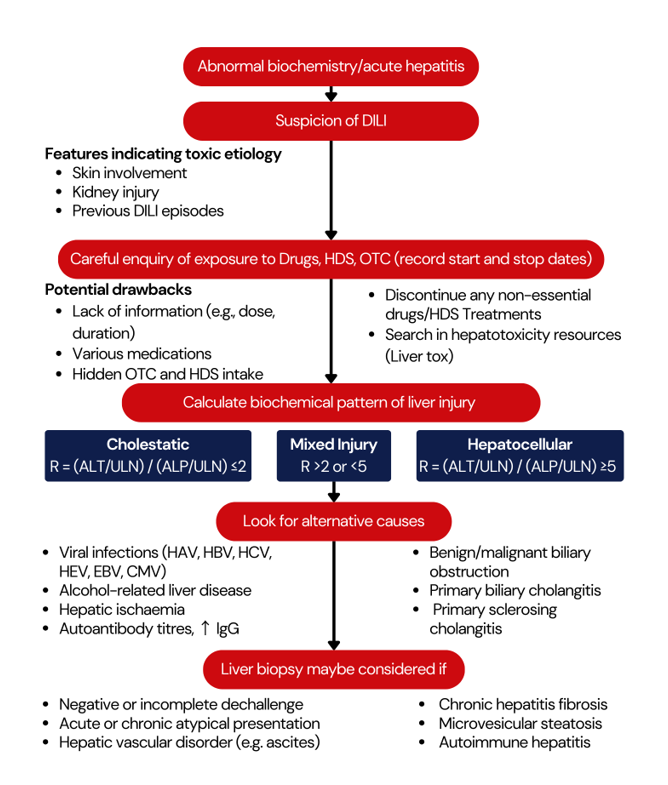
Conclusion:
- DILI is a key concern for regulators, drug developers, and physicians, and is difficult to predict during drug development process.
- As severe DILI is generally rare, finding a single case may require treatment of thousands of people from varied patient populations.
- The clinical trials present an exclusive opportunity to detect hepatotoxicity and cases of potential DILI with a study drug prior to its use in general population.
- Monitoring the liver test abnormalities is useful for assessing trends over time and to analyse imbalance between study drug and placebo/comparator groups.
- Due to the limited number of subjects in a clinical trial, monitoring the standard serum liver tests to detect milder liver injury can be considered a predominant approach to predict the risk of possible DILI in clinical trials.
- Considering that there may be varied mechanisms of DILI and different clinicopathological phenotypes, a systematic collection of adequate diagnostic datasets along with a focused causality assessment performed by clinical experts is required for evaluation of each potential case of DILI in clinical trials.
References
- EASL Clinical Practice Guidelines: Drug-induced liver injury. J Hepatol (2019), https://doi.org/10.1016/j.jhep.2019.02.014
- Evolution of the Food and Drug Administration Approach to Liver Safety Assessment for New Drugs: Current Status and Challenges. John R. Senior. Drug Saf (2014) 37 (Suppl 1):S9–S17
- Drug-induced liver injury (DILI): Current status and future directions for drug development and the post-market setting. A consensus by a CIOMS Working Group. Geneva, Switzerland: CIOMS, 2020
- Guidance for Industry Drug Induced Liver Injury: Premarketing Clinical Evaluation. U S. Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER).
Center for Biologics Evaluation and Research (CBER). July 2009. Drug Safety - EASL Clinical Practice Guidelines: Drug-induced liver injury. Journal of Hepatology 2019, Vol. 70, Issue 6, P1222–1261.
- Evolution of the Food and Drug Administration Approach to Liver Safety Assessment for New Drugs: Current Status and Challenges. Drug Saf (2014) 37 (Suppl 1):S9–S17
- Management of Safety Information from Clinical Trials. Report of CIOMS Working Group VI. Geneva, Switzerland: 2005.
- Guidance for Industry (Draft). Sponsor Responsibilities – Safety Reporting Requirements and Safety Assessment for IND and
Bioavailability/Bioequivalence Studies. FDA June 2021. - European Medicines Agency. Guideline on good pharmacovigilance practices (GVP) Module IX – Signal management (Rev 1). 09 October 2017.
About Soterius
Soterius is a strong team of pharma professionals who design customized, innovative, and cost-efficient processes for clinical safety, pharmacovigilance, and medical affairs. Our deep industry knowledge and up to date insights let us combine agile, people powered intelligence in pioneering customer centric solutions. Our innovative technology solutions include engagement tools and communications platforms to create a unified and compliant medical access facility. With a strong global presence, we provide comprehensive clinical and post marketed safety services, that include aggregate report writing, signal detection and management, global literature surveillance, risk management, case processing and regulatory reporting. We use state-of-the-art technologies to solve complex safety operations problems, be it case processing, intake, site reporting for clinical trials, or literature search and management. We have one of the most accurate solutions for case intake and case processing using AI.
We support companies from the initial development stage of a drug/vaccine to the approval and ultimate marketing of the therapy, supporting ongoing operations and regulatory commitments globally.
