
Medical Literature Monitoring
The medical literature is a vital source of information for monitoring the safety and benefit-risk profile of medicinal products. It is a significant source of information of suspected adverse reaction case reports (also known as Individual Case Safety Reports (ICSRs).
The Medical Literature Monitoring (MLM) is a service provided by the European Medicines Agency (EMA) for a number of medicinal products with multiple marketing authorisations and many marketing authorisation holders (MAHs), to identify suspected adverse reactions (ICSRs). The EMA is also responsible for entering the relevant information (identified from the MLM service) into the EudraVigilance database. The MAHs are not required to report to EudraVigilance, the suspected adverse reactions recorded in the listed medical literature for products being monitored by EMA. It is important to note that MAHs shall however, monitor all other medical literature not covered by MLM service, and report any suspected adverse reactions.
The purpose of the MLM service is to:

Medical Literature Monitoring by the European Medicines Agency
Active Substances Monitored by EMA
The EMA monitors a variety of active substances in the medicinal products for which a large number of authorisations were granted to various MAHs. The list of active substances monitored is published by the EMA on a specific webpage of the EMA website titled ‘MLM Substance and Herbal Substance Groups’.
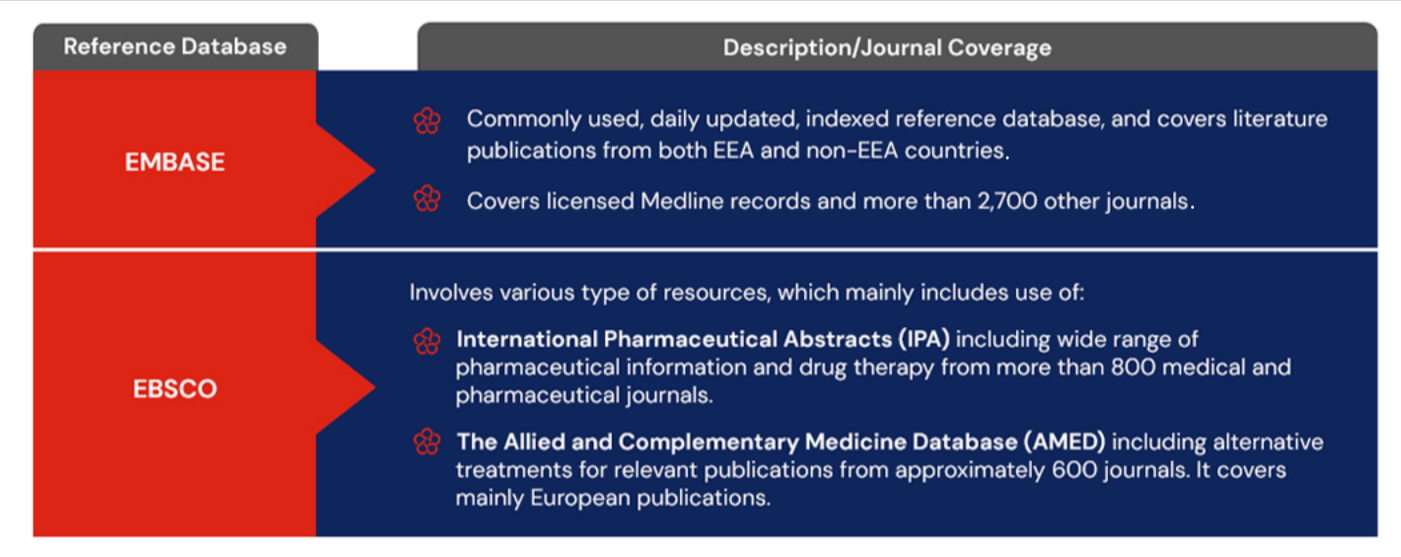
Medical Literature Databases/Journals used by EMA
The EMA employs daily updated, extensive, comprehensive, and indexed reference literature databases for performing literature search activities. These literature reference databases along with their journal coverage is mentioned below:
Medical and Scientific Literature Search by the EMA
Frequency of Literature Search by the EMA
- Daily search – The indexed biomedical reference database is searched daily; daily refers to the calendar days except the weekends (Saturday and Sunday).
- Monthly search– Two references databases focusing on pharmaceutical information and drug therapy as well as alternative treatments and complimentary medicine are searched monthly.
Search Strategies
EMA customize the search strategies for each substance group based on specific strings and publish the strategy on a specific webpage of the EMA website titled ‘MLM Search Strategies’. In order to enhance precision of the search, the search strategy is updated, as required. The updates are also evident in the ‘MLM Search Strategies’.
Search Results
The next calendar day after the search is conducted, the search results are published at a specific area of the EudraVigilance website. The key elements of the search results include name of substance group, the reference database used, time and date of conducting the search, title of the publication, name of the author(s), name of the journal etc.
Screening and Assessment of Medical Literature and Recording of Activities
Within one calendar day of the execution of literature search, EMA performs a review and preliminary assessment of each record.
The aim of screening and assessment procedure is to recognize valid Individual Case Safety Reports (ICSRs) relating to:
- Suspected adverse reactions (spontaneous reports or solicited reports).
- Special situations such as use of a medicinal product during pregnancy or breastfeeding, paediatric or elderly population.
- Reports of off-label use, misuse, overdose, medication errors, lack of therapeutic effect, lack of efficacy etc.
The ICSRs refer to suspected serious adverse reactions occurring both within and outside the EU and suspected non-severe adverse reactions occurring within the EU.
To simplify the screening process and make it efficient, inclusion/exclusion criteria are used by the EMA. These criteria are periodically reviewed and modified, as appropriate, and are made available on a specific webpage of the EMA website.
The publication records that do not meet the requirements for ICSR reporting are moved to an exclusion group, with the exclusion criteria noted. The records that might be eligible for ICSR reporting are moved to an inclusion group, where a duplicate check is performed. The records are then grouped into those that may refer to either as confirmed ICSRs or as potential ICSRs based on the criteria for valid ICSRs. For records of potential ICSRs, the full text publication (and if required, an English translation) is obtained and reviewed with the inclusion/exclusion criteria. Publications which do not qualify for a valid ICSR are moved to the exclusion group after recording the exclusion criteria. The results of these records after screening are published by the EMA on a specific webpage titled ‘MLM Search Results’.
The concerned MAH can access the ICSRs identified by the EMA (by the MLM service) from the EudraVigilance database.
They can also be downloaded in an Extensible Markup Language (XML) format.
There are documented quality controls to ascertain promptness, accuracy, and thoroughness of the literature screening, review, and the assessment process.
In order to assure the safety and effectiveness of medicines, global literature monitoring is a crucial part of pharmacovigilance. Pharmacovigilance teams can discover and assess potential adverse drug reactions and other safety issues early by keeping an eye on a variety of literature sources for potential safety concerns, which is essential for preserving patient health and ensuring the success of drug development programmes.
References
- European Medicines Agency: Guideline on good pharmacovigilance practices (GVP) Module VI – Collection, management and submission of reports of suspected adverse reactions to medicinal products (Rev 2); Dated 28 July 2017.
- European Medicines Agency: Detailed guide regarding the monitoring of medical literature and the entry of relevant information into the EudraVigilance database by the European Medicines Agency; Dated 12 May 2015
- European Medicines Agency: Monitoring of medical literature and the entry of relevant information into the EudraVigilance database by the European Medicines Agency; Description of the Journal/Reference databases used; Dated 21 December 2016
About Soterius
Soterius is a strong team of pharma professionals who design customized, innovative, and cost-efficient processes for clinical safety, pharmacovigilance, and medical affairs. Our deep industry knowledge and up to date insights let us combine agile, people powered intelligence in pioneering customer centric solutions. Our innovative technology solutions include engagement tools and communications platforms to create a unified and compliant medical access facility. With a strong global presence, we provide comprehensive clinical and post marketed safety services, that include aggregate report writing, signal detection and management, global literature surveillance, risk management, case processing and regulatory reporting. We use state-of-the-art technologies to solve complex safety operations problems, be it case processing, intake, site reporting for clinical trials, or literature search and management. We have one of the most accurate solutions for case intake and case processing using AI.
We support companies from the initial development stage of a drug/vaccine to the approval and ultimate marketing of the therapy, supporting ongoing operations and regulatory commitments globally.
