
Pharmacovigilance for Decentralized Clinical Trials
Pharmacovigilance for Decentralized Clinical Trials: Challenges and Way Forward
Decentralised clinical trials make clinical trials easier for patients by reducing the need to travel to clinical sites. They are also known as “Direct-to-participant trials” or “virtual” studies.
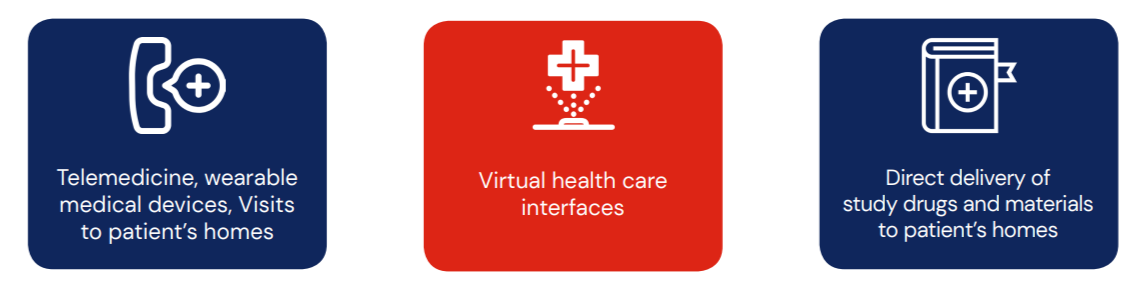
DCTs are highly technology driven that often require the use of the following:
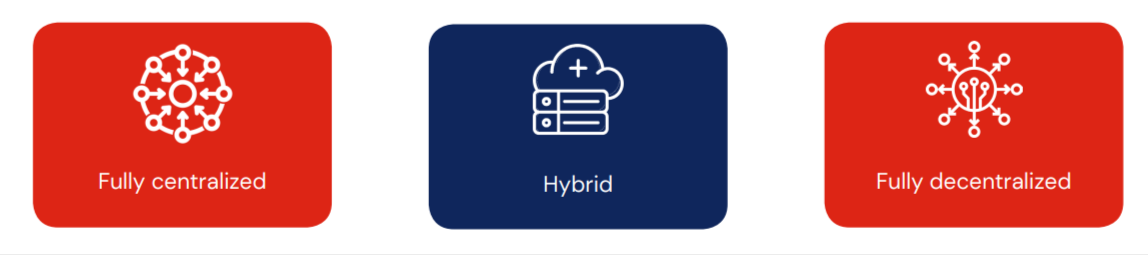
Depending upon the clinical trial design and practicality, DCTs may be:
Challenges posed by multiple data systems and processing teams
- Challenges in consolidation of data at the time of document preparation.
- Reconciliation of data can potentially take longer.
- Submission delays.
- Inspections & Audits become more complex.
- Vendor management is complex and more expensive.
- Partner Notifications/Exchange of Information, additional tracked activities.
Requirements of the Centralized Safety System
A Centralized Safety System requires the following key elements to cater to the challenging requirements of ensuring prompt monitoring of safety:
- Technical Agreement
- Safety Management Plans
- Central SOPs with Work Instructions
- Site Communication Protocol
- Safety Database + Processes
- Compliance and Governance
- Validated Safety Database System
- EDC <> Safety Data Exchange
- Secure Notifications to Sites
- Follow Ups and Site Queries Tracking Tools
- Literature Management Tools
- Signal and Trending Tools, Volume Dependent
- AI Based Tools to process large volumes of data
- Data Migration Tools to support product transfers, etc
About Soterius
Copyright 2025 by Soterius, Inc. All rights reserved. Soterius logo are trademarks or registered trademarks of Soterius in all jurisdictions. Other marks may be trademarks or registered trademarks of their respective owners. The information you see, hear or read on the pages within this presentation, as well as the presentation’s form and substance, are subject to copyright protection. In no event, may you use, distribute, copy, reproduce, modify, distort, or transmit the information or any of its elements, such as text, images or concepts, without the prior written permission of Soterius. No license or right pertaining to any of these trademarks shall be granted without the written permission of Soterius (and any of its global offices and/or affiliates). Soterius reserves the right to legally enforce any infringement of its intellectual property, copyright and trademark rights. Any content presented herewith should only be considered for general informational purposes and should not be considered as specific to the requirements of any particular organisation or for any specific purpose. Soterius does not make any representations or warranties about the completeness, reliability, appropriateness, relevance, or accuracy of the content presented here.
Confidential Information
Copyright@2025
Soterius, Inc.
