CONTACT OUR SALES
WE ARE SOTERIUS
Strong team of experienced professionals from the pharma industry. We design customized, innovative, and cost-efficient processes for clinical safety, pharmacovigilance, and medical affairs. Our deep industry knowledge and up to date insights let us combine agile, people powered intelligence with big data in pioneering customer centric solutions.




Soterius is a global industry leader in providing comprehensive drug safety and medical affairs services.
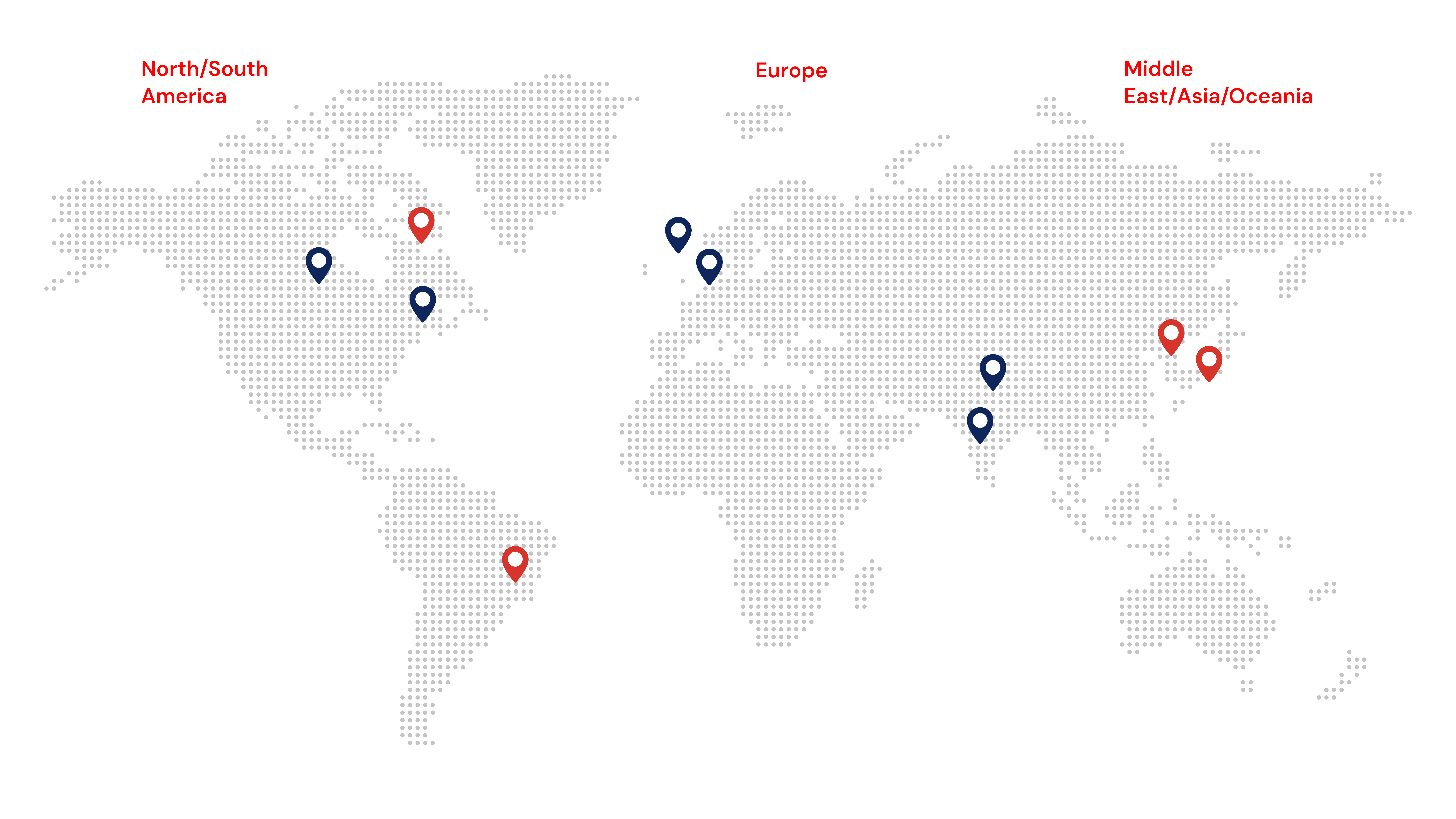
Our group employs 300+ strong team, serving top pharma companies globally, with safety experts, QPPVs, and Local Safety Managers in 60+ countries. We offer full end-to-end pharmacovigilance services, including case intake, processing, signal detection, aggregate report writing, and various technology solutions that improve compliance and operational efficiency in Pharmacovigilance.
We are a customer-focused organization, with a proven commitment to finding solutions for the people it serves, to driving excellence in compliance, and to improving patient outcomes. Soterius has its headquarters in New Jersey and offices in North America, Europe, and Asia.
Our quality systems are compliant, we are 24×7 available and always inspection ready.
Global Expertise
Group Offices
Partner Offices

Our Principles
Our Team of Experts

Suneet Walia
CEO, Chairman of the Board

Sumit Verma
Director and President, Operations Management, CSPV

Sonia Veluchamy
Board Member

Rakesh Dighe
Board Member

Howard Abroms
Vice President, Global Business Development

Suneel Gupta
Advisory Board

Barton Cobert
Advisory Board

Hal Ward
Advisory Board

Liia Ramachandra
Advisory Board

Arun Purohit
Advisory Board

Bharti Khanna
Advisory Board

Tanvi Chaturvedi
Associate Director, PV Operations & Technology

Yogesh Gulati
Senior Director, Clinical Safety and Pharmacovigilance

Pratibha Sharma
Subject Matter Expert, Clinical Safety and Pharmacovigilance

Wendy Kleijberg
QPPV Designate

Sonia Ariana Afsari
Medical Monitor

Bhumi Vyas
Head – Corporate Quality Assurance