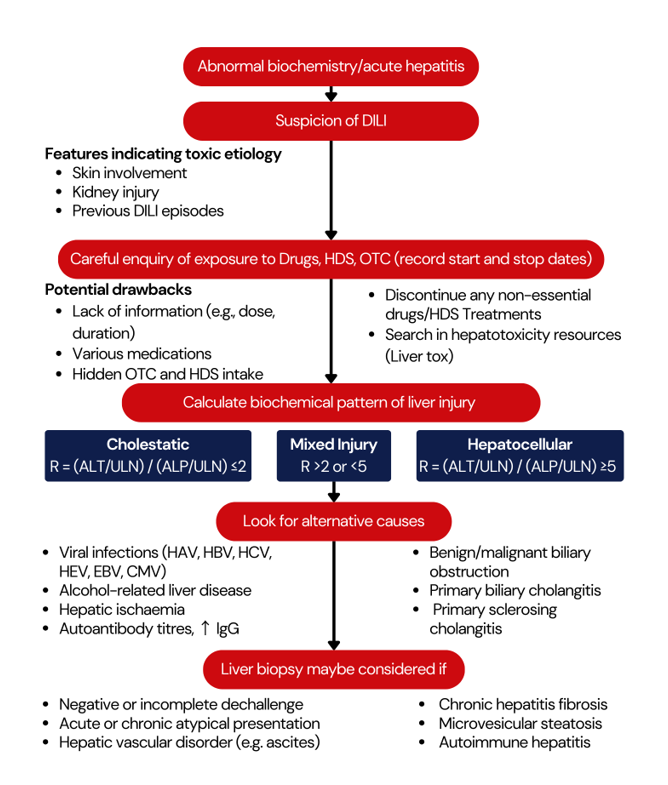
Introduction
EudraVigilance is a centralised European database of suspected adverse reactions to medicines that are authorised or being studied in clinical trials in the European Economic Area (EEA).
EudraVigilance is the system for managing and analysing information on suspected adverse reactions to medicines which have been authorised or being studied in clinical trials in the European Economic Area (EEA). The European Medicines Agency (EMA) operates the system on behalf of the European Union (EU) medicines regulatory network.
EudraVigilance supports safe and effective use of medicines by facilitating:
- Electronic exchange of ICSRs (Individual Case Safety Reports) between EMA, NCAs, MAHs and CT Sponsors in the EEA
- Early detection and evaluation of possible safety signals
- Better product information for medicines authorised in the EEA.
This electronic reporting is mandatory for marketing authorisation holders and sponsors of clinical trials. Marketing authorisation holders and sponsors of clinical trials must report and evaluate suspected adverse drug reactions during the development and following the marketing authorisation of medicinal products in the European Economic Area (EEA). Marketing authorisation holders must also electronically submit information on medicinal products authorised in the European Union (EU).
Why do we need a Responsible Person for EudraVigilance (RPEV)?
The Qualified Person for Pharmacovigilance (QPPV, for MAHs), or the Responsible Person (RP, for Sponsors/ Non-Commercial Sponsors) is responsible for managing an organization and its users in the EudraVigilance Production system.
For an individual to provide RP services to a sponsor/non-commercial sponsor, any one user in the individual’s organization should successfully complete the EudraVigilance ICSR knowledge evaluation and XEVMPD knowledge evaluation. To ensure the quality of data submitted to the EMA, the EMA offers training courses on ICSR and Product submissions using EVWeb, after which users may undergo knowledge evaluations.
Registration of Responsible Person in EudraVigilance
Registration of a Responsible Person could entail one of the following two scenarios:
Scenario 1: Change of RP (when there is still an RP in the organization), or
Scenario 2: New RP registration (when the first user of a new organization is registering as RP)
A set of documents must be submitted to the EMA to register the Responsible Person in either of the above scenarios.
| Documents Required | Scenario 1
(Change of RP) |
Scenario 2
(New RP) |
| Cover letter from the headquarters lever of the organization on a headed paper. The Cover letter should be signed by the new RP of by a person in a position above that at the headquarters leverl= (i.e., director of the organization or similar), or by the legal representative or Commercial and Non-Commercial Sponsors. | The cover letter should state the name and position of the previous RP and the name, position and contact details of the new RP. | The cover letter should state the name, position and contact details of the new RP. |
| Email Confirmation from the OMS Data Stewards acknowledging the successful creation of the new organization. | Not required, as the organization is already registered with OMS by the
older RP. |
Required |
| Copy of the ID card or driver’s license or passport, with the full name and signature visible. Any other information contained on the ID document may be blacked out. | Required | Required |
| Documents Required | Scenario 1
(Change of RP) |
Scenario 2
(New RP) |
| User declaration form for RP, including the type and same of the organization, user’s details, and dated and signed by the user | Required | Required |
| EudraVigilance ICSR and XEVMPD submission training. | A declaration from the QPPV/RP that the organization has a suitably trained person or submission of ICSRs and XEVPRMs. This declaration can be included in the cover letter or in the body of the email submitted via the EV Registration Service Desk. Submitting copies of ICSR and XEVMPD Certificates is not necessary when changing the QPPV/RP. | A copy of the notification of successful completion of the EudraVigilance ICSR
and XEVMPD knowledge evaluation for at least one user to access the production environment, as applicable |
| Form A- for Sponsor based
in the EEA. Signed by the Sponsor’s legal representative person appointing the new responsible person for clinical trials, including the name and the contact details of this person. The legal representative person and responsible person address should be for the respective organizations the work for. |
Required | Required |
| Form B- for Sponsor’s based outside the EEA only. Signed by someone from the Sponsor appointing the Sponsor’s legal
representative person in the EEA, including the name and the contact details of this person. |
Required | Required
The legal representative person address should be for the organization the legal representative works for. |
| A EudraCT number for a study the sponsor is conducting. | Required | Required |
Conclusion
We hope that this blog was helpful in understanding the role of a Responsible Person and the process of registration of a RP with the EMA. Please reach out to us if you require RP services in the EEA or wish to understand more about the registration process for the RP.