Pharmacovigilance for Decentralized Clinical Trials: Challenges and Way Forward
Decentralised clinical trials make clinical trials easier for patients by reducing the need to travel to clinical sites. They are also known as “Direct-to-participant trials” or “virtual” studies.
DCTs are highly technology driven that often require the use of the following:
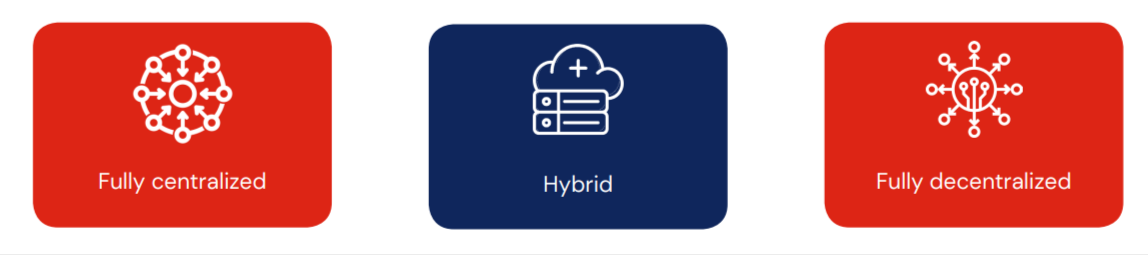
Depending upon the clinical trial design and practicality, DCTs may be:
Challenges posed by multiple data systems and processing teams
- Challenges in consolidation of data at the time of document preparation.
- Reconciliation of data can potentially take longer.
- Submission delays.
- Inspections & Audits become more complex.
- Vendor management is complex and more expensive.
- Partner Notifications/Exchange of Information, additional tracked activities.
Requirements of the Centralized Safety System
A Centralized Safety System requires the following key elements to cater to the challenging requirements of ensuring prompt monitoring of safety:
- Technical Agreement
- Safety Management Plans
- Central SOPs with Work Instructions
- Site Communication Protocol
- Safety Database + Processes
- Compliance and Governance
- Validated Safety Database System
- EDC <> Safety Data Exchange
- Secure Notifications to Sites
- Follow Ups and Site Queries Tracking Tools
- Literature Management Tools
- Signal and Trending Tools, Volume Dependent
- AI Based Tools to process large volumes of data
- Data Migration Tools to support product transfers, etc
